Copper-catalyzed asymmetric allylic alkylation of racemic inert cyclic allylic ethers under batch and flow conditions†
Abstract
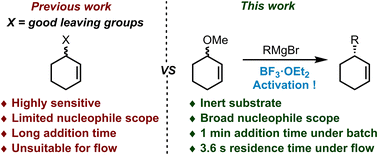
Highly enantioselective Cu-catalyzed asymmetric allylic alkylation of racemic inert cyclic allylic ethers is accomplished in this work. The use of Grignard reagents in combination with BF3·OEt2 and CuBr·SMe2/L2 is the key to enable employment of long-challenging low reactive allylic substrates in this AAA reaction. In addition, the approach exhibited a remarkable superiority in the construction of a stereogenic center involving challenging sterically hindered nucleophiles for both primary and secondary alkyl groups. Extension of this method under continuous flow conditions was also performed with excellent enantioselectivity in a short residence time. Mechanistic experiments indicated that the reaction proceeded through an in situ generated allylic bromide intermediate. All these advantages demonstrated that this chiral catalytic system is a valuable complement for existing asymmetric catalytic methods.


 Please wait while we load your content...
Please wait while we load your content...