Regioselective C–H alkylation of anisoles with olefins by cationic imidazolin-2-iminato scandium(iii) alkyl complexes†
Abstract
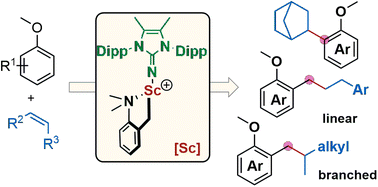
A new type of rare-earth alkyl complexes supported by monoanionic imidazolin-2-iminato ligands were synthesised and structurally characterised by X-ray diffraction and NMR analyses. The utility of these imidazolin-2-iminato rare-earth alkyl complexes in organic synthesis was demonstrated by their performance in highly regioselective C–H alkylation of anisoles with olefins. With as low as 0.5 mol% catalyst loading, various anisole derivatives without ortho-substitution or 2-methyl substituted anisoles reacted with several alkenes under mild conditions, producing the corresponding ortho-Csp2-H and benzylic Csp3-H alkylation products in high yield (56 examples, 16–99% yields). Control experiments revealed that rare-earth ions, ancillary imidazolin-2-iminato ligands, and basic ligands were crucial for the above transformations. Based on deuterium-labelling experiments, reaction kinetic studies, and theoretical calculations, a possible catalytic cycle was provided to elucidate the reaction mechanism.
- This article is part of the themed collection: 2023 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...