Biomimetic chlorine-induced polyene cyclizations harnessing hypervalent chloroiodane–HFIP assemblies†
Abstract
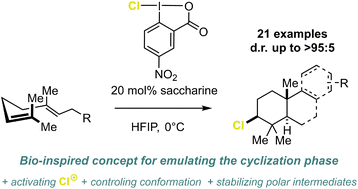
While bromo- and iodocyclizations have recently been successfully implemented, the challenging chlorocyclizations have been scantly investigated. We present a selective and generally applicable concept of chlorination-induced polyene cyclization by utilizing HFIP–chloroiodane networks mimicking terpene cyclases. A manifold of different alkenes was converted with excellent selectivities (up to d.r. >95 : 5). The cyclization platform was even extended to several structurally challenging terpenes and terpenoid carbon frameworks.


 Please wait while we load your content...
Please wait while we load your content...