The spatial distribution of cobalt phthalocyanine and copper nanocubes controls the selectivity towards C2 products in tandem electrocatalytic CO2 reduction†
Abstract
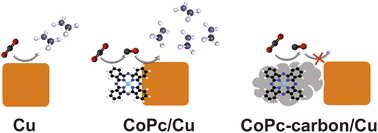
The coupling of CO-generating molecular catalysts with copper electrodes in tandem schemes is a promising strategy to boost the formation of multi-carbon products in the electrocatalytic reduction of CO2. While the spatial distribution of the two components is important, this aspect remains underexplored for molecular-based tandem systems. Herein, we address this knowledge gap by studying tandem catalysts comprising Co-phthalocyanine (CoPc) and Cu nanocubes (Cucub). In particular, we identify the importance of the relative spatial distribution of the two components on the performance of the tandem catalyst by preparing CoPc-Cucub/C, wherein the CoPc and Cucub share an interface, and CoPc-C/Cucub, wherein the CoPc is loaded first on carbon black (C) before mixing with the Cucub. The electrocatalytic measurements of these two catalysts show that the faradaic efficiency towards C2 products almost doubles for the CoPc-Cucub/C, whereas it decreases by half for the CoPc-C/Cucub, compared to the Cucub/C. Our results highlight the importance of a direct contact between the CO-generating molecular catalyst and the Cu to promote C–C coupling, which hints at a surface transport mechanism of the CO intermediate between the two components of the tandem catalyst instead of a transfer via CO diffusion in the electrolyte followed by re-adsorption.
- This article is part of the themed collections: Most popular 2023 catalysis articles and 2023 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...