Enantioselective rhodium-catalyzed addition of arylboronic acids to N-heteroaryl ketones: synthesis of α-hydroxy acids†
Abstract
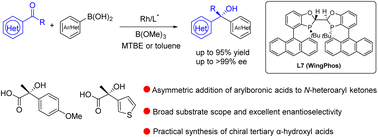
The enantioselective addition of arylboronic acids to N-heteroaryl ketones provides a convenient access to chiral α-heteroaryl tertiary alcohols, yet addition reactions of this type have been challenging due to catalyst deactivation. In this report, an efficient rhodium-catalyzed addition of arylboronic acids to N-heteroaryl ketones is established, affording a variety of valuable α-heteroaryl alcohols with excellent functional group compatibility. The employment of the WingPhos ligand containing two anthryl groups is crucial for this transformation. In particular, a range of chiral benzoxazolyl-substituted tertiary alcohols were formed with excellent ee values and yields by employing a Rh loading as low as 0.3 mol%, which can serve as a practical protocol to furnish a series of chiral α-hydroxy acids after hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...