Manipulating D–A interaction to achieve stable photoinduced organic radicals in triphenylphosphine crystals†
Abstract
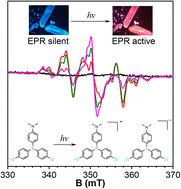
New strategies for the design and synthesis of stable organic radicals without additives are highly desirable. Herein, we design a series of donor–acceptor structured triarylphosphines and disclose the fast color change triggered by UV-irradiation in the crystalline state. Photoinduced organic radicals are undoubtedly verified and proved to be the reason for the color change by time-dependent and quantitative electron paramagnetic resonance analysis, X-ray crystallographic analysis, and theoretical calculations. It is revealed that the intrinsic symmetry breaking of peripheral architecture helps to form continuous molecular chains by donor–acceptor counterpart pairing. Intermolecular electron-transfer occurs among molecular chains and results in radical ion pairs upon photoirradiation.


 Please wait while we load your content...
Please wait while we load your content...