Rate and equilibrium constants for the addition of triazolium salt derived N-heterocyclic carbenes to heteroaromatic aldehydes†
Abstract
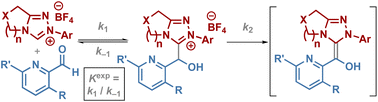
Heteroaromatic aldehydes are often used preferentially or exclusively in a range of NHC-catalysed processes that proceed through the generation of a reactive diaminoenol or Breslow Intermediate (BI), with the reason for their unique reactivity currently underexplored. This manuscript reports measurement of rate and equilibrium constants for the reaction between N-aryl triazolium NHCs and heteroaromatic aldehydes, providing insight into the effect of the NHC and heteroaromatic aldehyde structure up to formation of the BI. Variation in NHC catalyst and heteroaromatic aldehyde structure markedly affect the observed kinetic parameters of adduct formation, decay to starting materials and onward reaction to BI. In particular, large effects are observed with both 3-halogen (Br, F) and 3-methyl substituted pyridine-2-carboxaldehyde derivatives which substantially favour formation of the tetrahedral intermediate relative to benzaldehyde derivatives. Key observations indicate that increased steric hindrance leads to a reduction in both k2 and k−1 for large (2,6-disubstituted)-N-Ar groups within the triazolium scaffold, and sterically demanding aldehyde substituents in the 3-position, but not in the 6-position of the pyridine-2-carboxaldehyde derivatives. As part of this study, the isolation and characterisation of twenty tetrahedral adducts formed upon addition of N-aryl triazolium derived NHCs into heteroaromatic aldehydes are described. These adducts are key intermediates in NHC-catalysed umpolung addition of heteroaromatic aldehydes and are BI precursors.


 Please wait while we load your content...
Please wait while we load your content...