Next-generation membrane-active glycopeptide antibiotics that also inhibit bacterial cell division†
Abstract
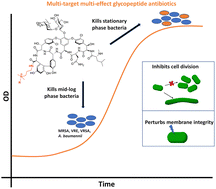
Resistance to vancomycin, a life-saving drug against Gram-positive bacterial infections necessitates developing alternative therapeutics. Herein, we report vancomycin derivatives that assimilate mechanisms beyond D-Ala–D-Ala binding. The role of hydrophobicity towards the structure and function of the membrane-active vancomycin showed that alkyl-cationic substitutions favored broad-spectrum activity. The lead molecule, VanQAmC10 delocalized the cell division protein MinD in Bacillus subtilis, implying an impact on bacterial cell division. Further examination of wild-type, GFP-FtsZ, or GFP-FtsI producing- and ΔamiAC mutants of Escherichia coli revealed filamentous phenotypes and delocalization of the FtsI protein. The findings indicate that VanQAmC10 also inhibits bacterial cell division, a property previously unknown for glycopeptide antibiotics. The conjunction of multiple mechanisms contributes to its superior efficacy against metabolically active and inactive bacteria, wherein vancomycin is ineffective. Additionally, VanQAmC10 exhibits high efficacy against methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii in mouse models of infection.


 Please wait while we load your content...
Please wait while we load your content...