Acene enlargement for absorption red-shifting and photosensitization enhancement of photosensitizers with aggregation-induced emission†
Abstract
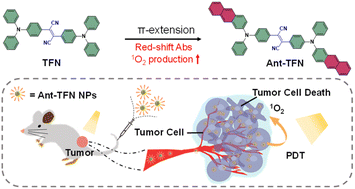
Photosensitizers with aggregation-induced emission (AIE PSs) were widely explored in photodynamic therapy. Numerous acceptors but few donors were reported to design AIE PSs. In this study, we developed a new kind of donor that can improve the comprehensive performance of AIE PSs by expanding the π extension of aromatic rings at the end of the triphenylamine group through acene enlargement. The absorption and fluorescence peaks of anthryl-substituted AIE PS are red-shifted by 29 nm and 42 nm; the photosensitization efficiency is enhanced by 1.16 times; the AIE factor is 86.1 and the fluorescence quantum yield is 9.3%. We also demonstrated that the anthryl-based AIE PS can image and ablate cancer cells well both in vitro and in vivo. The anthryl-triphenylamine donor provides an excellent option to design donor–acceptor AIE PSs with high comprehensive performance.


 Please wait while we load your content...
Please wait while we load your content...