Nitrogen-doped polycyclic aromatic hydrocarbons by a one-pot Suzuki coupling/intramolecular SNAr reaction†
Abstract
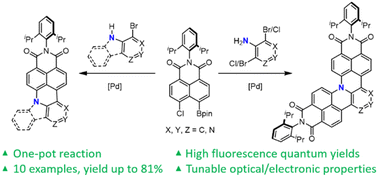
We report a new method for the synthesis of nitrogen-doped (N-doped) polycyclic aromatic hydrocarbons (PAHs) by a Suzuki coupling/intramolecular SNAr cascade reaction. A one- or two-fold [3 + 3] naphtho-annulation of halogenated aniline was conducted under Suzuki–Miyaura cross-coupling conditions to yield a series of fully fused N-doped PAHs. In contrast to reported methods to synthesize pyridinic or pyrrolic nitrogen-doped PAHs, our method enables preparation of PAHs doped with graphitic nitrogen, for which few reports are known in the literature. The crystal structure as well as absorption, fluorescence and electrochemical properties of these N-doped PAHs were investigated, which demonstrated the capability of N-doping to adjust optical and electronic properties and alter the LUMO energy level.


 Please wait while we load your content...
Please wait while we load your content...