Orthogonal, modular anion–cation and cation–anion self-assembly using pre-programmed anion binding sites†
Abstract
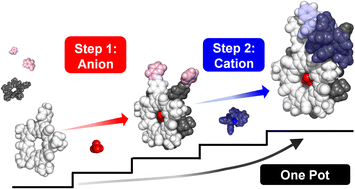
Subcomponent self-assembly relies on cation coordination whereas the roles of anions often only emerge during the assembly process. When sites for anions are instead pre-programmed, they have the potential to be used as orthogonal elements to build up structure in a predictable and modular way. We explore this idea by combining cation (M+) and anion (X−) binding sites together and show the orthogonal and modular build up of structure in a multi-ion assembly. Cation binding is based on a ligand (L) made by subcomponent metal-imine chemistry (M+ = Cu+, Au+) while the site for anion binding (X− = BF4−, ClO4−) derives from the inner cavity of cyanostar (CS) macrocycles. The two sites are connected by imine condensation between a pyridyl-aldehyde and an aniline-modified cyanostar. The target assembly [LM-CS-X-CS-ML],+ generates two terminal metal complexation sites (LM and ML) with one central anion-bridging site (X) defined by cyanostar dimerization. We showcase modular assembly by isolating intermediates when the primary structure-directing ions are paired with weakly coordinating counter ions. Cation-directed (Cu+) or anion-bridged (BF4−) intermediates can be isolated along either cation–anion or anion–cation pathways. Different products can also be prepared in a modular way using Au+ and ClO4−. This is also the first use of gold(I) in subcomponent self-assembly. Pre-programmed cation and anion binding sites combine with judicious selection of spectator ions to provide modular noncovalent syntheses of multi-component architectures.
- This article is part of the themed collection: #MyFirstChemSci 2023


 Please wait while we load your content...
Please wait while we load your content...