The kinked structure and interchain van der Waals interaction of carbyne nanocrystals†
Abstract
Carbyne with one-dimensional sp-hybridized carbon atoms is the third form of carbon following diamond and graphite. Although carbyne nanocrystals have been synthesized, little is known about its structural details. Here, we report experimental evidence of the kinked structure of carbon chains and interchain van der Waals interaction of carbyne nanocrystals by near edge X-ray absorption fine structure (NEXAFS) spectroscopy. We measure the 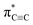 resonance and the feature peaks of the kinked configuration of carbon chains and the van der Waals interaction between chains of carbyne nanocrystals using NEXAFS spectroscopy. We also perform theoretical calculations of density functional theory and simulations based on the super-cell core-hole method for carbon K-edge NEXAFS. The theoretical results are in good agreement with the experimental measurements, which demonstrates that carbyne nanocrystals are van der Waals crystals with kinked chains as structural units. Note that the peak at 288.5 eV in the simulated NEXAFS spectrum implies the possible presence of hydrogen-terminated kinks or hydrogen-terminated chains in carbyne nanocrystals, which clarifies the understanding of the C–H bond in carbyne nanocrystals. These findings are enlightening and significant for pursuing physics and potential applications of carbyne.
resonance and the feature peaks of the kinked configuration of carbon chains and the van der Waals interaction between chains of carbyne nanocrystals using NEXAFS spectroscopy. We also perform theoretical calculations of density functional theory and simulations based on the super-cell core-hole method for carbon K-edge NEXAFS. The theoretical results are in good agreement with the experimental measurements, which demonstrates that carbyne nanocrystals are van der Waals crystals with kinked chains as structural units. Note that the peak at 288.5 eV in the simulated NEXAFS spectrum implies the possible presence of hydrogen-terminated kinks or hydrogen-terminated chains in carbyne nanocrystals, which clarifies the understanding of the C–H bond in carbyne nanocrystals. These findings are enlightening and significant for pursuing physics and potential applications of carbyne.



 Please wait while we load your content...
Please wait while we load your content...