A versatile strategy for the formation of hydride-bridged actinide–iridium multimetallics†
Abstract
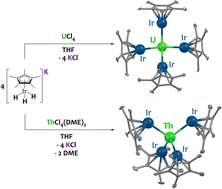
Reaction of the potassium pentamethylcyclopentadienyl iridate tris-hydride K[IrCp*H3] with UCl4 and ThCl4(DME)2 led to the complete replacement of the halide ligands to generate multimetallic complexes U{(μ-H)3IrCp*}4 (1) and Th{[(μ-H2)(H)IrCp*]2[(μ-H)3IrCp*]2} (2), respectively. These analogues feature a significant discrepancy in hydride bonding modes; 1 contains twelve bridging hydrides while 2 contains ten bridging hydrides and two terminal, Ir-bound hydrides. Use of a U(III) starting material, UI3(1,4-dioxane)1.5, resulted in the octanuclear complex {U[(μ2-H3)IrCp*]2[(μ3-H2)IrCp*]}2 (3). Computational studies indicate significant bonding character between U/Th and Ir in 1 and 2, with f-orbital involvement in the singly-occupied molecular orbitals of the uranium species 1. In addition, these studies attribute the variation in hydride bonding between 1 and 2 to differences in dispersion effects.


 Please wait while we load your content...
Please wait while we load your content...