Discovery of a simple iron catalyst reveals the intimate steps of C–H amination to form C–N bonds†
Abstract
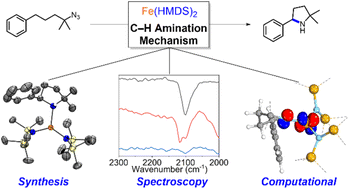
Formation of ubiquitous C–N bonds traditionally uses prefunctionalized carbon precursors. Recently, metal-catalyzed amination of unfunctionalized C–H bonds with azides has become an attractive and atom-economic strategy for C–N bond formation, though all catalysts contain sophisticated ligands. Here, we report Fe(HMDS)2 (HMDS = N(SiMe3)2−) as an easy-to-prepare catalyst for intramolecular C–H amination. The catalyst shows unprecedented turnover frequencies (110 h−1vs. 70 h−1 reported to date) and requires no additives. Amination is successful for benzylic and aliphatic C–H bonds (>80% yield) and occurs even at room temperature. The simplicity of the catalyst enabled for the first time comprehensive mechanistic investigations. Kinetic, stoichiometric, and computational studies unveiled the intimate steps of the C–H amination process, including the resting state of the catalyst and turnover-limiting N2 loss of the coordinated azide. The high reactivity of the iron imido intermediate is rationalized by its complex spin system revealing imidyl and nitrene character.


 Please wait while we load your content...
Please wait while we load your content...