Diversity-oriented synthesis of medium-sized cyclophanes via the photo-fries rearrangement of N-aryl lactams†
Abstract
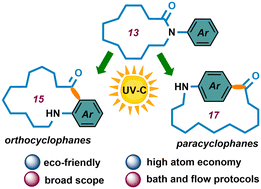
Macrocycles containing a cyclophane skeleton have intrigued scientists for many years due to their sophisticated structure and wide use in materials science and molecular recognition processes. Preparing macrocycles with a cyclophane skeleton, however, is inherently difficult due to the high entropic cost of ring-closing. Herein, we report a green and sustainable protocol for the preparation of structurally diverse cyclophane skeletons via a light-promoted intramolecular Fries-type rearrangement, starting from simple N-aryl lactams. This operationally straightforward reaction proceeds with excellent atom economy, solely under light irradiation without any catalysts or additives and provides a practical route to ortho- and para-cyclophanes with never-seen-before architectures.


 Please wait while we load your content...
Please wait while we load your content...