“Domino” synthesis of bio-derived anethole over facile-prepared hafnium phosphonate frameworks with efficient bifunctional acid sites†
Abstract
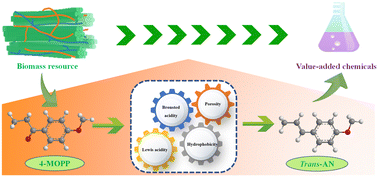
Utilization of renewable biomass resources to transform them into value-added platform molecules has aroused widespread attention. Anethole (AN) is widely considered to be a significant chemical owing to its extensive application in food additives, daily necessities and pharmaceuticals. Therefore, reducing the dependence on natural fennel oil is a rational approach. We report a “green” and effective pattern of stereoselective conversion of biomass-derived 4′-methoxyphenylacetone (4-MOPP) into trans-AN. We employed a “domino” strategy of catalytic transfer hydrogenation and dehydration promoted by an interesting Lewis/Brønsted bifunctional acid catalyst, which was developed by template-free self-assembly of Hf4+ and phenylphosphonic acid (PA) followed by grinding sulfonation. Importantly, by simply adjusting composite components, PA–Hf–SO3H (1.5 : 1) exhibited a favorable amorphous inorganic–organic structure with specific surface area (135.59 m2 g−1), affluent mesopores (4.01 nm), indispensable Lewis/Brønsted acidic sites, as well as strong hydrophobicity and stability. The optimal reaction conditions were investigated by the single-factor method and response surface methodology; a high yield of 98.3% with excellent reusability was demonstrated. The corresponding kinetic and thermodynamic parameters were determined, and a feasible reaction mechanism proposed. Finally, the developed model showed a wide range of substrate versatility, thereby providing a promising catalytic strategy for the effective synthesis of AN or other high-value molecules.


 Please wait while we load your content...
Please wait while we load your content...