Reaction pathways and kinetics of N-acetyl-d-glucosamine hydrolysis in sub- and supercritical water†
Abstract
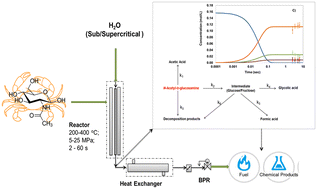
Subcritical and supercritical water hydrolysis of N-acetyl-D-glucosamine (NAG) to value-added chemicals has been studied in detail. Experiments were carried out using a continuous flow reactor by varying temperature (250–400 °C), pressure (5–25 MPa) and residence time (2–60 s). A wide product distribution was obtained during the reactions, including solid, liquid and gaseous compounds, sensitive to the state of water: sub- or supercritical. Subcritical conditions promoted the formation of solid compounds, whereas gasification was favored in supercritical water. The prominent products present in the liquid fraction were glycolic acid, acetic acid, formic acid, 5-HMF and acetamide. The formation of a few distinct nitrogen-containing heterocyclic compounds was observed only in supercritical water. Following instantaneous deacetylation of NAG, several oxidation, reduction and secondary reactions occur during hydrolysis of NAG in subcritical and supercritical water. A kinetic model for the prediction of yields of acetic acid, glycolic acid and formic acid is developed. The network of reactions showed specifc deviations from conventional understanding. The current findings show that it is possible to develop a suitable process to make a set of value-added chemicals from chitinous biomass.


 Please wait while we load your content...
Please wait while we load your content...