Impact of solvent sulfolane in enhancing methanol selectivity during methane partial oxidation on Fe-ZSM5 catalyst with H2O2 as an oxidant†
Abstract
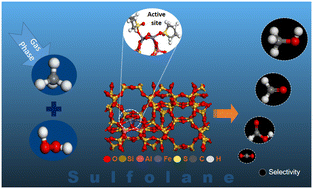
The liquid-phase direct partial oxidation of methane to methanol is highly desirable for methane utilization. Using a combination of density functional theory and molecular dynamics simulations, this investigation reports the impact of sulfolane solvent on the reaction mechanism of methane oxidation with H2O2 in Fe-ZSM-5 catalyst and the potential product distribution. Sulfolane favored the formation of [Fe–OH] and [Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] as active oxygen species from H2O2 dissociation, unlike Fe-OOH in water. On [Fe–OH], methanol formation was via a methoxy intermediate (activation barrier, Ea = 1.79 eV), while on [Fe
O] as active oxygen species from H2O2 dissociation, unlike Fe-OOH in water. On [Fe–OH], methanol formation was via a methoxy intermediate (activation barrier, Ea = 1.79 eV), while on [Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O], it was by the radical rebound mechanism (Ea = 1.41 eV). The formation of formaldehyde was kinetically and thermodynamically less favorable than methanol and the formation of formic acid had a higher barrier. The lower diffusivity of H2O2 than CH3OH and the lower diffusivity of these in sulfolane than in water, together with the trends in reaction barriers, indicate higher methanol selectivity in sulfolane than in water.
O], it was by the radical rebound mechanism (Ea = 1.41 eV). The formation of formaldehyde was kinetically and thermodynamically less favorable than methanol and the formation of formic acid had a higher barrier. The lower diffusivity of H2O2 than CH3OH and the lower diffusivity of these in sulfolane than in water, together with the trends in reaction barriers, indicate higher methanol selectivity in sulfolane than in water.


 Please wait while we load your content...
Please wait while we load your content...