C–H activation and subsequent C–C bond formation in rigid alkenes catalyzed by Ru(iii) metallates†
Abstract
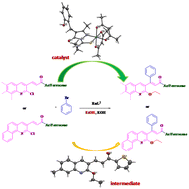
Pyrazolone derived Ru(III) complexes were synthesized and comprehensively characterized by spectral techniques. The true nature of the complexes was revealed by X-ray crystallography in which the ligand coordinated in a monobasic bidentate fashion. The catalytic efficiency of the complexes was analyzed in the C–H activation reaction of α,β-unsaturated carbonyl compounds. The reactions proceeded well in ethanol with potassium hydroxide as a base. In addition to Heck type coupling at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, ethanol mediated ethoxylation of the C–Cl bond at the C2 position of the quinoline moiety was witnessed and validated by establishing the structure of a representative intermediate through X-ray diffraction analysis. All the catalytic products were characterized by 1H and 13C NMR analysis and representatives by mass spectral studies.
C bond, ethanol mediated ethoxylation of the C–Cl bond at the C2 position of the quinoline moiety was witnessed and validated by establishing the structure of a representative intermediate through X-ray diffraction analysis. All the catalytic products were characterized by 1H and 13C NMR analysis and representatives by mass spectral studies.


 Please wait while we load your content...
Please wait while we load your content...