Synthesis and evaluation of potent (iso)ellipticine-based inhibitors of MYLK4 accessed via expeditious synthesis from isoquinolin-5-ol†
Abstract
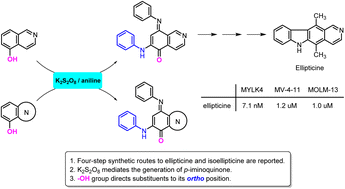
The K2S2O8-mediated generation of p-iminoquinone contributed to the regioselective substitution of isoquinolin-5,8-dione. This hydroxyl group-guided substitution was also applied to selected heterocycles and addressed the regioselectivity issue of quinones. This study has provided an expeditious pathway from isoquinolin-5-ol (5) to ellipticine (1) and isoellipticine (2), which benefits the comprehensive comparison of their activity. Compounds 1 and 2 displayed marked MYLK4 inhibitory activity with IC50 values of 7.1 and 6.1 nM, respectively. In the cellular activity of AML cells (MV-4-11 and MOLM-13), compound 1 showed better AML activity than compound 2.


 Please wait while we load your content...
Please wait while we load your content...