Rapid assembly of structurally diverse cyanamides and disulfanes via base-mediated aminoalkylation of aryl thiourea†
Abstract
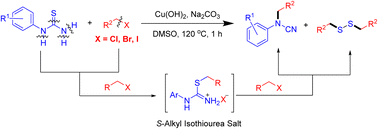
A general method for the preparation of cyanamides and disulfanes from aryl thiourea and halide through a base-mediated strategy is described. Mercaptan and N-aryl cyanamide are the key intermediates in the reaction. The current method is convenient, eco-friendly, and has high yields for the synthesis of substituted cyanamide and functional disulfanes in a one-pot procedure from readily available starting materials.


 Please wait while we load your content...
Please wait while we load your content...