Elucidating arsenic-bound proteins in the protein data bank: data mining and amino acid cross-validation through Raman spectroscopy†
Abstract
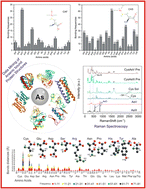
The International Agency for Research on Cancer has unequivocally classified inorganic arsenic as a Group 1 carcinogen, definitively establishing its potential to induce cancer in humans. Paradoxically, despite its well-documented toxicity, arsenic finds utility as a chemotherapeutic agent. Notable examples include melarsoprol and arsenic trioxide, both employed in the treatment of acute promyelocytic leukemia. In both therapeutic and hazardous contexts, arsenic can accumulate within cellular environments, where it engages in intricate interactions with protein molecules. Gaining a comprehensive understanding of how arsenic compounds interact with proteins holds immense promise for the development of innovative inhibitors and pharmaceutical agents. These advancements could prove invaluable in addressing a spectrum of arsenic-related diseases. In pursuit of this knowledge, we undertook a systematic exploration of the Protein Data Bank, with a focus on 902 proteins intricately associated with 26 arsenic compounds. Our comprehensive investigation reveals insights into the interactions between these arsenical compounds and amino acids located within a 4.0 Å molecular distance from arsenic-binding sites. Our findings identify that cysteine, glutamic acid, aspartic acid, serine, and arginine frequently engage with arsenic. In complement to our computational analyses, we conducted rigorous Raman spectroscopy studies on the top five amino acids displaying robust interactions with arsenic. The results derived from experimental Raman spectroscopy were meticulously compared with our computational assessments, thereby enhancing the reliability and depth of our investigations. The current study presents a multidimensional exploration into the elaborate interplay between arsenic compounds and proteins. By elucidating the specific amino acids that preferentially interact with arsenic, this study not only contributes to the fundamental understanding of these molecular associations but also lays the foundation for future endeavors in drug design and therapeutic interventions targeting arsenic-related illnesses. Our work at the convergence of toxicology, medicine, and molecular biology carries profound implications for advancing our knowledge of arsenic's dual nature as both a poison and a potential cure.


 Please wait while we load your content...
Please wait while we load your content...