Adsorption isotherms and kinetics for Pb(ii) ion removal from aqueous solutions with biogenic metal oxide nanoparticles†
Abstract
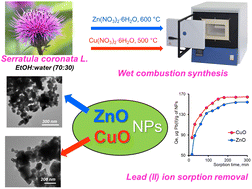
This study investigates the sorption removal of lead(II) ions using zinc oxide (ZnO) and copper(II) oxide (CuO) nanoparticles synthesized through a wet burning method with the aid of plant extract from Serratula coronata L. The effect of plant collection time on polyphenol content was investigated and optimal conditions were determined. The structural and chemical properties of the nanoparticles were studied by scanning electron microscopy, energy dispersive analysis, X-ray phase analysis, and X-ray photoelectron spectroscopy. A comparative analysis of lead ion sorption on the surface of synthesized nanoparticles was conducted. The kinetic study revealed that the sorption process follows a pseudo-second-order mechanism, and the Freundlich sorption model provides a better fit for the experimental data. ZnO and CuO nanoparticles exhibited significant sorption capacities, with values of 163.6 and 153.8 mg g−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...