Research status of soda residue in the field of environmental pollution control
Abstract
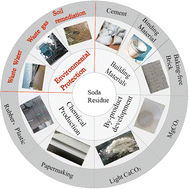
High-quality soda ash (Na2CO3) is mainly produced using the ammonia–alkaline method, generating a significant amount of industrial waste called soda residue. In China, the annual production of soda residue exceeds 10 million tons. The large-scale open-air storage of soda residue not only occupies land but also causes severe pollution to the surrounding environment. Soda residue displays characteristics such as strong alkalinity, high reactivity, and a well-developed pore structure, making it a valuable raw material for producing environmentally functional materials. This article provided an overview and summary of soda residue, including its sources and hazards, basic properties, applications in environmental management (wastewater treatment, flue gas desulfurization, and soil remediation), and associated risks. The limitations of using soda residue in “waste to waste” technologies were also analyzed. Based on this analysis, the article suggests focusing on simultaneous removal of heavy metal ions using soda residue, safely disposing of and acquiring resources from metal-laden sludge, efficiently dechlorinating soda residue, using soda residue for contaminated soil solidification, stabilization, and assisted remediation, controlling pollution via green and circular utilization approaches, and assessing long-term risk.
- This article is part of the themed collection: 2023 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...