Novel practical stereoselective synthesis of a bicyclic hydantoino-thiolactone as the key intermediate for production of (+)-biotin†‡
Abstract
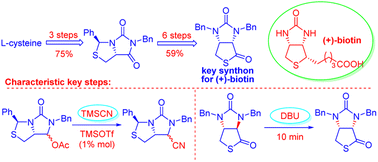
Bicyclic hydantoinothiolactone (1), as the key intermediate for production of (+)-biotin, has been efficiently and high-stereoselectively synthesized from the cheap starting material L-cystine via nine steps in 44% overall yield. In this new practical synthesis, there are two characteristic steps worthy of note. One step is TMSOTf-catalyzed efficient cyanation of (3S,7aR)-6-benzyl-5-oxo-3-phenyltetrahydro-1H,3H-imidazo[1,5-c]thiazol-7-yl acetate, the other step is DBU-catalyzed rapid isomerization of trans-isomer to cis-isomer of the bicyclic hydantoinothiolactone.
- This article is part of the themed collection: 2023 RSC Advances Popular Advances Collection


 Please wait while we load your content...
Please wait while we load your content...