DFT approach towards accurate prediction of 1H/13C NMR chemical shifts for dipterocarpol oxime†
Abstract
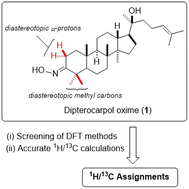
A computational NMR approach for accurate predicting the 1H/13C chemical shifts of triterpenoid oximes featuring the screening of 144 DFT methods was demonstrated. Efficiently synthesized dipterocarpol oxime was employed as a model compound. The six highest accurate methods from the screening generated root-mean-square-error (RMSE) values in the range of 0.84 ppm (0.55%) to 1.14 ppm (0.75%) for calculated 13C shifts. For 1H results, simple, economical 6-31G basis set unexpectedly outperformed other more expensive basic sets; and the couple of it with selected functionals provided high accuracy shifts (0.0617 ppm (1.49%) ≤ RMSE ≤ 0.0870 ppm (2.04%)). These computational results strongly supported the proton and carbon assignments of the oxime including the difficult ones of diastereotopic methyl groups, the methyl groups attached to an internal olefin, and diastereotopic α-protons.


 Please wait while we load your content...
Please wait while we load your content...