Combating drug-resistant bacteria with sulfonium cationic poly(methionine)†
Abstract
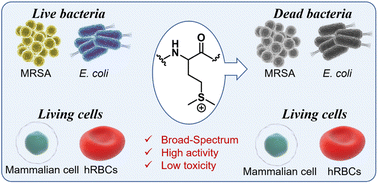
Abstract antibiotic resistance and drug-resistant bacterial infections pose significant threats to public health. Antimicrobial peptides (AMPs) are a promising candidate for related-infection therapy, but their clinical application is limited by their high synthesis cost and susceptibility to protease degradation. To address these issues, cationic poly(α-amino acid)s based on lysine have been developed as synthetic mimics of AMPs. In this study, we introduce a new class of cationic AMP synthetic mimics based on functional poly(methionine)s. We synthesized a series of sulfonium cationic poly(D,L-methionine)s with varying chain lengths via a convenient polymerization on α-amino acid thiocarboxyanhydride (α-NTA) using tert-butyl-benzylamine as the initiator, followed by alkylation with iodomethane. Our optimal methionine polymer demonstrated potent and broad-spectrum antibacterial activity against antibiotic-resistant bacteria, as well as excellent biocompatibility with mammalian cells and rapid bactericidal performance. Our findings suggest that sulfonium poly(methionine)s have the potential to address the challenge of drug-resistant bacterial infections.


 Please wait while we load your content...
Please wait while we load your content...