Convergent micro-wave assisted synthesis of quinazolinone and its precursor using the bio-sourced solvent pinane†
Abstract
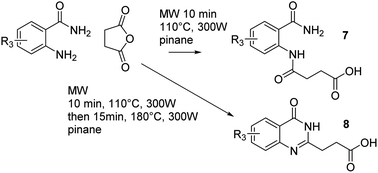
A general microwave synthesis of 4-oxo-3,4-dihydroquinazolin-2-yl propanoic acids and their diamide precursors from the corresponding substituted benzamide and succinic anhydride is described, using pinane as a sustainable solvent that favors the cyclization step. The conditions are some of the most simple and cost effective reported.

 Please wait while we load your content...
Please wait while we load your content...