An efficient method to access spiro pseudoindoxyl ketones: evaluation of indoxyl and their N-benzylated derivatives for inhibition of the activity of monoamine oxidases†
Abstract
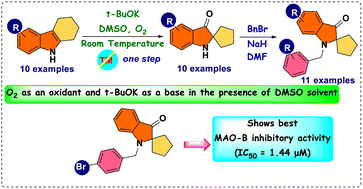
A simple, metal-free approach was developed to obtain novel pseudoindoxyl derivatives. The reaction was mediated by tBuOK on tetrahydrocarbazole 8 in dimethyl sulfoxide (DMSO) at room temperature through the hydroxylation of the indole double bond and a subsequent pinacol-type rearrangement. Spiro pseudoindoxyl compounds and their N-benzylated derivatives were assessed for their inhibitory activities against monoamine oxidase (MAO) enzymes. Based on half-maximal inhibitory concentration (IC50) values, 13 compounds were found to have higher inhibitory activity against MAO-B than against MAO-A. With regard to MAO-B inhibition, 11f showed the best inhibitory activity, with an IC50 value of 1.44 μM, followed by 11h (IC50 = 1.60 μM), 11j (IC50 = 2.78 μM), 11d (IC50 = 2.81 μM), and 11i (IC50 = 3.02 μM). Compound 11f was a competitive inhibitor with a Ki value of 0.51 ± 0.023 μM. In a reversibility experiment using dialysis, 11f showed effective recovery of MAO-B inhibition similar to that of safinamide. These experiments suggested that 11f was a potent, reversible, and competitive inhibitor of MAO-B activity.


 Please wait while we load your content...
Please wait while we load your content...