Rapid synthesis of functional poly(ester amide)s through thiol–ene chemistry†
Abstract
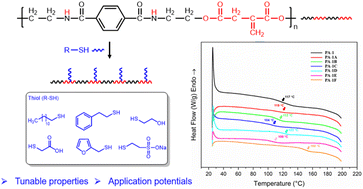
Poly(ester amide)s (PEAs) bearing various side chains were synthesized by post-polymerization modification of PA-1, a vinylidene containing PEA. The thiols 1-dodecanethiol (1A-SH), 2-phenylethanethiol (1B-SH), 2-mercaptoethanol (1C-SH), thioglycolic acid (1D-SH), furfuryl mercaptan (1E-SH) and sodium-2-mercaptoethanesulfonate (1F-SH) were reacted with PA-1 to form PEAs PA-1A through PA-1F respectively. PEAs containing non-polar thiol side chains (PA-1A, PA-1B, PA-1E), showed little change in solubility compared to PA-1, while PEAs with more polar side chains improved solubility in more polar solvents. PA-1F, functionalized with sodium-2-mercaptoethanesulfonate, became water-soluble. The introduction of pendant functional groups impacted the thermal behaviors of PEAs in a wide range. The PEAs were thermally stable up to 368 °C, with glass transition temperatures (Tg) measured between 117 to 152 °C. Moreover, to demonstrate the versatility of the PEAs, thermal reprocessable networks and polyurethanes were successfully fabricated by reacting with a bismaleimide (1,6-bis(maleimido)hexane, 1,6-BMH) and a diisocyanate (4,4′-diphenylmethane diisocyanate, 4,4′-MDI), respectively. This study paves the way for the facile synthesis of functional poly(ester amide)s with great potential in many fields.


 Please wait while we load your content...
Please wait while we load your content...