Facile synthesis of new N-(aminocycloalkylene)amino acid compounds using chiral triflate esters with N-Boc-aminopyrrolidines and N-Boc-aminopiperidines†
Abstract
In this study, we prepared a series of new N-(aminocycloalkylene)amino acid derivatives for use in chiral building blocks. The method was based on the conversion of enantiopure α-hydroxy acid esters into the corresponding chiral triflate esters, which were displaced by a nucleophilic substitution SN2 reaction with aminopyrrolidine and aminopiperidine derivatives, and the inversion of the configuration to give methyl 2-[(Boc-amino)cycloamin-1-yl]alkanoates with good yield and high enantiomeric and diastereomeric purity. Synthesized 2-[(Boc-amino)piperidin-1-yl]propanoates combined with ethyl L-phenylalaninate gave new chiral N-Boc- and N-nosyl-dipeptides containing a piperidine moiety. The structures were elucidated by 1H-, 13C-, and 15N-NMR spectroscopy, high-resolution mass spectrometry, and X-ray crystallography analyses.
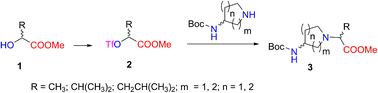


 Please wait while we load your content...
Please wait while we load your content...