Efficient enzymatic synthesis of chiral 2,3-dihydro-1,4-benzodioxane motif using engineered Candida antarctica lipase B†
Abstract
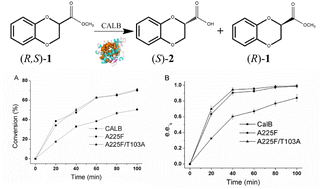
Chiral motifs of 2,3-dihydro-1,4 benzodioxane are extensively utilized in diverse medicinal substances and bioactive natural compounds, exhibiting significant biological activities. Notable examples of such therapeutic agents include prosympal, dibozane, piperoxan, and doxazosin. In this work, using 1,4-benzodioxane-2-carboxylic acid methyl ester as the substrate, after screening 38 CALB covariant residues, we found that mutants A225F and A225F/T103A can catalyze the kinetic resolution of the substrate. The effect of temperature, cosolvent, and cosolvent concentration on kinetic resolution was investigated, revealing that the best results were achieved at 30 °C with 20% n-butanol as a cosolvent, resulting in an optimal resolution (e.e.s 97%, E = 278) at 50 mM substrate concentration. Structure analysis showed that mutation sites 225 and 103 are not among the sites that interact directly with the substrate, which means that covariant amino acids that interact remotely with the substrate also regulate enzyme catalysis. This research may provide us with a new strategy for enzyme evolution.


 Please wait while we load your content...
Please wait while we load your content...