Synthesis and biological evaluation of novel β-lactam-metallo β-lactamase inhibitors†
Abstract
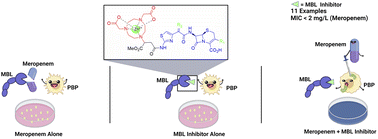
β-lactamases are enzymes that deactivate β-lactam antibiotics through a hydrolysis mechanism. There are two known types of β-lactamases: serine β-lactamases (SBLs) and metallo β-lactamases (MBLs). The two existing strategies to overcome β-lactamase-mediated resistance are (a) to develop novel β-lactam antibiotics that are not susceptible to hydrolysis by these enzymes; or (b) to develop β-lactamase inhibitors that deactivate the enzyme and thereby restore the efficacy of the co-administered antibiotics. Many commercially available SBL inhibitors are used in combination therapy with antibiotics to treat antimicrobial resistant infections; however, there are only a handful of MBL inhibitors undergoing clinical trials. In this study, we present 11 novel potential MBL inhibitors (via multi-step chemical synthesis), that have shown to completely restore the efficacy of meropenem (≤2 mg L−1) against New Delhi metallo-β-lactamase (NDM) producing Klebsiella pneumoniae in vitro. These compounds contain a cyclic amino acid zinc chelator conjugated to various commercially available β-lactam antibiotic scaffolds with the aim to improve the overall drug transport, lipophilicity, and pharmacokinetic/pharmacodynamic properties as compared to the chelator alone. Biological evaluation of compounds 24b and 24c has further highlighted the downstream application of these MBLs, since they are non-toxic at the selected doses. Time-kill assays indicate that compounds 24b and 24c exhibit sterilizing activity towards NDM producing Klebsiella pneumoniae in vitro using minimal concentrations of meropenem. Furthermore, 24b and 24c proved to be promising inhibitors of VIM-2 (Ki = 0.85 and 1.87, respectively). This study has revealed a novel series of β-lactam MBLIs that are potent, efficacious, and safe leads with the potential to develop into therapeutic MBLIs.


 Please wait while we load your content...
Please wait while we load your content...