NIR squaraine dyes for dual colorimetric and fluorescent determination of Fe3+, Cu2+, and Hg2+ ions†
Abstract
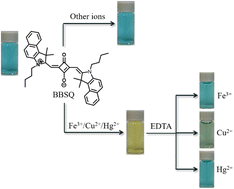
Four benzoindolenine-based squaraine dyes (SQs), which have the advantages of intense visible and near-infrared (NIR) absorption and emission (λabs/max 663–695 nm, λem/max 686–730 nm) were synthesized and characterized by UV-vis absorption, fluorescent emission spectrophotometry, FTIR, NMR and HRMS analysis. Among them, BBSQ showed excellent performance, which exhibited high selectivity to Fe3+, Cu2+, and Hg2+ in acetonitrile solution even in the presence of other competitive metal ions, accompanied by obvious color change easily detected by the naked eye. The detection limit was 14.17 μM for Fe3+ and 6.06 μM for Cu2+. Most importantly, the response mechanism of BBSQ to Fe3+, Cu2+, and Hg2+ involves the coordination of BBSQ and metal ions through the O atom on the central squarate ring, N atom, and olefin π bond of BBSQ and has been demonstrated by Job's plot, FTIR, and 1H NMR titration analyses. Furthermore, BBSQ was applied successfully to detect Fe3+, Cu2+, and Hg2+ in thin-layer chromatography (TLC) plates with good precision and is quite promising for the quantitative detection of Fe3+ and Cu2+ ions in water samples.


 Please wait while we load your content...
Please wait while we load your content...