Highly regioselective and diastereoselective synthesis of novel pyrazinoindolones via a base-mediated Ugi-N-alkylation sequence†
Abstract
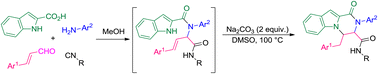
An efficient base-mediated/metal-free approach has been developed for the synthesis of 1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide derivatives via intramolecular indole N–H alkylation of novel bis-amide Ugi-adducts. In this protocol the Ugi reaction of (E)-cinnamaldehyde derivatives, 2-chloroaniline, indole-2-carboxylic acid and different isocyanides was designed for the preparation of bis-amides. The main highlight of this study is the practical and highly regioselective preparation of new polycyclic functionalized pyrazino derivatives. This system is facilitated by Na2CO3 mediation in DMSO and 100 °C conditions.


 Please wait while we load your content...
Please wait while we load your content...