pH-dependent structural characteristics of lonidamine: 1H and 13C NMR study
Abstract
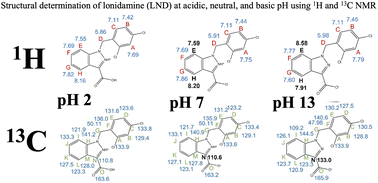
Lonidamine (LND) is an anti-cancer drug with great potential as a metabolic modulator of chemotherapy, radiotherapy, hyperthermia, and photodynamic therapy in cancer treatment. LND affects several important aspects of cancer cell metabolism: it inhibits Complex I and II of the electron transport chain (ETC) and pyruvate carriers (mitochondrial), and monocarboxylate transporters in the plasma membrane of the cell. Cancer cells are affected by changes in pH on the molecular level, and so are the drugs used to treat cancer, thus it is important to understand how pH affects their structures and LND is no exception. LND dissolves at a pH of 8.3 in tris-glycine buffer but has limited solubility at pH 7. To understand how pH affects the structure of LND, and its effect as a metabolic modulator on cancer therapy, we made up samples of LND at pH 2, pH 7, and pH 13, and analyzed these samples using 1H and 13C NMR. We looked for ionization sites to explain the behavior of LND in solution. Our results showed considerable chemical shifts between the extremes of our experimental pH range. LND was ionized at its indazole α-nitrogen, however, we did not directly observe the protonation of the carboxyl group oxygen that is expected at pH 2, which may be the result of a chemical-exchange phenomenon.


 Please wait while we load your content...
Please wait while we load your content...