Development of validated methods for the simultaneous quantification of Finasteride and Tadalafil in newly launched FDA-approved therapeutic combination: greenness assessment using AGP, analytical eco-scale, and GAPI tools†
Abstract
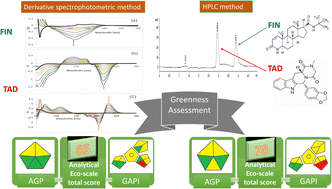
The primary objectives of green chemistry are the reduction of generation and use of hazardous substances. In healthcare, the most active areas of research in green chemistry are medication manufacturing and analysis. Analysts take serious steps for converting traditional analytical methods to eco-friendly ones that minimize the negative effects of solvents and chemicals on the environment and improve the healthcare. In the proposed work, two analytical methods are presented for the quantification of Finasteride (FIN) and Tadalafil (TAD) simultaneously in newly launched FDA-approved dosage form without prior separation. The first method is derivative spectrophotometry, which is based on measuring the amplitudes of first derivative spectrophotometric peaks of FIN and TAD in ethanolic solution at 221 nm and 293 nm, respectively. On the other hand, measuring the peak-to-peak amplitudes of second derivative spectrum of TAD solution at 291–299 nm was also performed. Regression equations show good linearity for FIN and TAD in the ranges of 10–60 μg mL−1 and 5–50 μg mL−1, respectively. The second method is the RP-HPLC method, where the chromatographic separation was achieved using the XBridgeTM C18 (150 × 4.6 mm, 5 μm) column. The eluent was the mixture of acetonitrile:phosphate buffer with triethylamine, 1% (v/v) adjusted to pH = 7 in the ratio of 50 : 50 (by volume). The flow rate was 1.0 mL min−1 with DAD-detection at 225 nm. This analytical procedure was linear over the ranges of 10–60 μg mL−1 and 2.5–40 μg mL−1 for FIN and TAD, respectively. The presented methods were validated (regarding ICH guidelines) and statistically compared by applying the t-test and F-test with the reported method. The greenness appraisal was performed using three different tools. The proposed validated methods were found to be green, sensitive, selective, and can be successfully used for quality control test.


 Please wait while we load your content...
Please wait while we load your content...