Bio-oriented synthesis of ibuprofen derivatives for enhancement efficacy in post-operative and chronic inflammatory pain models†
Abstract
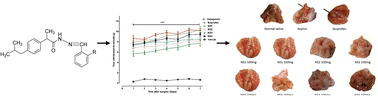
The discovery of post-operative, chronic inflammatory pain and any gastroulcerogenic potential using well-established animal models in vivo with new structures, high efficiency, broad-spectrum, and low toxicity has been the focus of medicinal chemists. In the present article, we are reporting the design and synthesis of various derivatives of ibuprofen by modifying the carboxyl group of ibuprofen using three steps reactions; esterification under microwave-irradiation in 10 minutes, hydrazide formation, and finally schiff's base reaction. Microwave-assisted esterification reaction can be employed to quickly explore and increase molecular diversity in synthetic chemistry. All of the newly synthesized compounds (NS1–NS4) were characterized by 1H-, 13C-NMR, and HR-ESI-MS spectroscopy and evaluated for post-operative, chronic inflammatory pain and any gastroulcerogenic potential using well-established animal models in vivo. The synthesized compounds at the tested doses of 100 and 150 mg kg−1 significantly attenuated the incisional-injury induced post-operative pain like condition and, also inhibited the phologistic agent induced inflammatory responses in both the acute and chronic testing paradigms. The gastric histological and biochemical parameters exhibited that the synthesized compounds were devoid of any ulcerogenic potential in comparison to aspirin and ibuprofen. These findings concluded that the synthesized ibuprofen derivatives exhibited profound analgesic, anti-inflammatory properties with reduced ulcerogenic potential and might be considered as effective therapeutic agents to treat pathological conditions associated with pain and inflammation.


 Please wait while we load your content...
Please wait while we load your content...