Synthesis of 2,5-dimethylhexene by isobutene dimerization with H2S co-feeding†
Abstract
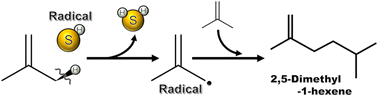
This study investigates the effect of hydrogen sulfide (H2S) co-feeding on the synthesis of 2,5-dimethyl-1-hexene, 2,5-dimethyl-2-hexene, and 2,5-dimethylhexane (2,5-DMHs), useful compounds, using the dimerization of isobutene under mild pressure conditions. The dimerization of isobutene did not proceed in the absence of H2S, whereas the desired products of 2,5-DMHs were produced under H2S co-feeding conditions. The effect of reactor size on the dimerization reaction was then examined, and the optimal reactor was discussed. To enhance the yield of 2,5-DMHs, we changed the reaction conditions of the temperature, molar ratio of isobutene to H2S (iso-C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /H2S) in the feed gas, and the total feed pressure. The optimum reaction condition was at 375 °C and 2/1 of iso-C4
/H2S) in the feed gas, and the total feed pressure. The optimum reaction condition was at 375 °C and 2/1 of iso-C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /H2S. The product of 2,5-DMHs monotonously increased by an increment of total pressure from 1.0 to 3.0 atm with a fixed iso-C4
/H2S. The product of 2,5-DMHs monotonously increased by an increment of total pressure from 1.0 to 3.0 atm with a fixed iso-C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) /H2S ratio at 2/1.
/H2S ratio at 2/1.


 Please wait while we load your content...
Please wait while we load your content...