Catalytic reactions of oxalic acid degradation with Pt/SiO2 as a catalyst in nitric acid solutions
Abstract
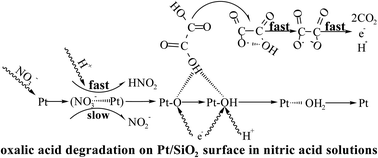
Large quantities of solutions containing oxalic acid and nitric acid are produced from nuclear fuel reprocessing, but oxalic acid must be removed before nitric acid and plutonium ions can be recovered in these solutions. The degradation of oxalic acid with Pt/SiO2 as a catalyst in nitric acid solutions has the characteristics of a fast and stable reaction, recyclable catalyst, and no introduction of impurity ions into the system. This method is one of the preferred alternatives to the currently used reaction of KMnO4 with oxalic acid but lacks theoretical support. Therefore, this study attempts to clarify the reaction mechanism of the method. First, there was no induction period for this catalytic reaction, and no evidence was found that the nitrous acid produced in the solution could have an effect on oxalic acid degradation. Furthermore, oxidation intermediates (structures of Pt–O) were formed through this reaction between NO3− adsorbed on the active sites and Pt on the catalyst surface, but H+ greatly promoted the reaction. Additionally, oxalic acid degradation through the oxidative dehydrogenation reaction occurred between oxalic acid molecules (HOOC–COOH) and Pt–O, with ·OOC–COOH, which is easily self-decomposable especially in acidic solution, generated simultaneously, and finally CO2 was produced.


 Please wait while we load your content...
Please wait while we load your content...