Synthesis and identification of new sacubitril derivatives as lead compounds for antibacterial, antifungal and antitubercular (TB) activities against dormant tuberculosis†
Abstract
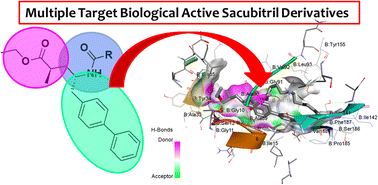
We identified twenty-two new sacubitril derivatives (5a–v) as lead compounds for various biologically active targets. These compounds were synthesized by reacting an intermediate compound (2R,4S)-5-([1,1′-biphenyl]-4-yl)-4-(amino)-2-methylpentanoic acid ethyl ester hydrochloride with respective carboxylic acid (RCOOH). The molecular structures of all the newly synthesized compounds were determined by 1H and 13C NMR, ESI mass spectrometry, FTIR spectroscopy, and CHN analysis. Moreover, compound 5n was characterized by a single-crystal X-ray diffraction (SXRD) study to confirm the structure obtained from spectral data. All these compounds were screened for various biological functions such as antifungal, antibacterial, and anti-TB activities. Among these twenty-two compounds (5a–v), some exhibited good to moderate anti-bacterial properties. Similarly, some compounds showed moderate anti-TB and antifungal activities. In addition, the anti-TB activity of compound 5q was estimated against M. tuberculosis in a nutrient starvation model (NSM). Similarly, toxicity was examined against RAW 264.7 cells. These biological activity studies were also correlated with molecular docking studies.


 Please wait while we load your content...
Please wait while we load your content...