Synthesis, structures and reactions of acylsulfenyl iodides with theoretical investigations†
Abstract
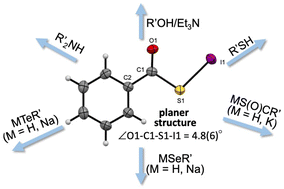
A series of acylsulfenyl iodides (RCOSI) were synthesized by the reactions of carbothioic acid group 11–16 element derivatives with iodine or N-iodosuccinimides in moderate to good yields. The structure of the PhCOSI was nearly square planar based on the X-ray analysis, where the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯I distance (3.153(5) Å) was significantly shorter than the sum of the van der Waals radii of the atoms (ΣrvdW), indicating close contact within the molecule. The distances between an iodine atom and the neighbouring two iodine atoms were also less than ΣrvdW, perhaps due to the energy lowering effect of the interactions. The acylsulfenyl iodides readily reacted with alkenes and alkynes to give the expected addition products in moderate to good yields at approximately 0 °C. A new synthesis of acylated sulfines, sulfenamides and sulfenochalcogenides using acylsulfenyl iodides is also described. Theoretical calculations were performed on PhCOSI with the Sapporo-TZP(+1s1p) basis sets at the MP2 level, which perfectly reproduced the observed structures. Similar calculations were performed on the reactions, exemplified by those of MeCOSI and CH2
O⋯I distance (3.153(5) Å) was significantly shorter than the sum of the van der Waals radii of the atoms (ΣrvdW), indicating close contact within the molecule. The distances between an iodine atom and the neighbouring two iodine atoms were also less than ΣrvdW, perhaps due to the energy lowering effect of the interactions. The acylsulfenyl iodides readily reacted with alkenes and alkynes to give the expected addition products in moderate to good yields at approximately 0 °C. A new synthesis of acylated sulfines, sulfenamides and sulfenochalcogenides using acylsulfenyl iodides is also described. Theoretical calculations were performed on PhCOSI with the Sapporo-TZP(+1s1p) basis sets at the MP2 level, which perfectly reproduced the observed structures. Similar calculations were performed on the reactions, exemplified by those of MeCOSI and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, together with those of MeSI and CH2
CH2, together with those of MeSI and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. Mechanisms for both reactions were proposed, which were very similar. The proposed mechanism for the former was understood based on that of the latter. For both mechanisms, the episulfuranes and episulfonium ions played an important role. The dynamic and static nature of the bonds in the COSI group of PhCOSI and MeCOSI were elucidated based on QTAIM dual functional analysis.
CH2. Mechanisms for both reactions were proposed, which were very similar. The proposed mechanism for the former was understood based on that of the latter. For both mechanisms, the episulfuranes and episulfonium ions played an important role. The dynamic and static nature of the bonds in the COSI group of PhCOSI and MeCOSI were elucidated based on QTAIM dual functional analysis.


 Please wait while we load your content...
Please wait while we load your content...