Engineering a Bacillus subtilis esterase for selective hydrolysis of d, l-menthyl acetate in an organic solvent-free system†
Abstract
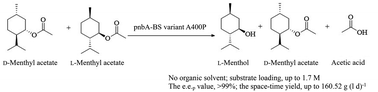
Esterase/lipase-catalyzed selective hydrolysis of D, L-menthyl esters has become one of the promising approaches for producing L-menthol, one of the most important flavoring chemicals with extensive uses. However, the activity and L-enantioselectivity of the biocatalyst are not sufficient for meeting the industrial requirements. Herein, a highly active para-nitrobenzyl esterase from Bacillus subtilis 168 (pnbA-BS) was cloned and then engineered to enhance its L-enantioselectivity. On the basis of the strategy tailoring the steric exclusion effect and structural flexibility of the region adjacent to the substrate, the substitution of Ala400 to Pro caused a remarkable improvement in the E value from 1.0 to 466.6. The variant A400P was purified and further confirmed with strict L-enantioselectivity in the selective hydrolysis of D, L-menthyl acetate, whereas the improved L-enantioselectivity caused decreased activity. To develop an efficient, easy-to-use, and green methodology, organic solvent was omitted and substrate constant feeding was integrated into the whole-cell catalyzed system. During the catalytic process, the selective hydrolysis of 1.0 M D, L-menthyl acetate in 14 h offered a conversion of 48.9%, e.e.p value of >99%, and space-time yield of 160.52 g (l d)−1.


 Please wait while we load your content...
Please wait while we load your content...