Efficient O-demethylation of lignin-derived aromatic compounds under moderate conditions†
Abstract
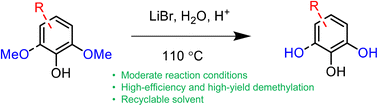
Lignin is a potential feedstock to produce renewable aromatic chemicals. However, lignin-derived aromatics are heavily methoxylated, which affects their reactivity in some downstream valorization attempts. Herein, we report an efficient method for the demethylation of the aromatics derived from lignin depolymerization using acidic concentrated lithium bromide (ACLB) under moderate conditions (e.g., 1.5 M HCl, 110 °C, and 2 h). Aromatics with one or two methoxy groups (G-type and S-type), alkyl hydroxyl and carbonyl groups, and electron-donating and electron-withdrawing substituents were used to investigate the demethylation mechanisms. S-type aromatics were demethylated faster than their G-type analogs. Alkyl hydroxyl groups were brominated under the conditions. Carbonyl groups (aldehydes and ketones) promoted unwelcome condensation. Electron-donating substituents promoted demethylation, whereas electron-withdrawing substituents retarded the demethylation. An ortho-carboxylic group enhanced the demethylation because of the formation of a stable intermediate.


 Please wait while we load your content...
Please wait while we load your content...