Low-cost diatomite supported binary transition metal sulfates: an efficient reusable solid catalyst for biodiesel synthesis†
Abstract
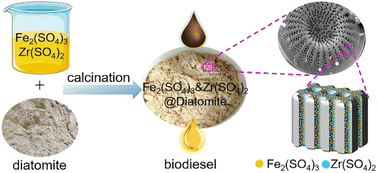
Using a simple method of impregnation and then calcination, diatomite supported binary transition metal sulfates (Fe and Zr, designated as Fe2(SO4)3&Zr(SO4)2@diatomite) were prepared and used as a catalyst in the preparation of renewable biofuels. The synthesised Fe2(SO4)3&Zr(SO4)2@diatomite catalyst (Fe2(SO4)3 : Zr(SO4)2 : diatomite = 1 : 2 : 6, mass ratio) was thoroughly characterised using transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, microbeam X-ray fluorescence (μ-XRF) spectroscopy and thermogravimetric analysis (TG). The results demonstrated that the sulfate was successfully loaded onto the diatomite with a uniform distribution. The N2 adsorption/desorption analysis indicated that the catalyst's specific surface area was 1.54 m2 g−1. The catalyst exhibited outstanding performance in the preparation of renewable biofuel (biodiesel) from waste fatty acids and the optimal parameters were methanol-to-oil 1.25 : 1, reaction temperature 70 °C, catalyst concentration 10 wt%, reaction time 4 h. The conversion was found to reach 98.90% under optimal parameters, which is better than that of Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite. Moreover, the catalyst can be recycled by simple filtration and reused for three cycles after regeneration without noticeable reduction in catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...