Rhodium-catalyzed selenylation and sulfenylation of quinoxalinones ‘on water’†
Abstract
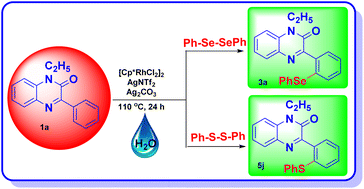
A rhodium-catalysed, regioselective synthetic methodology for selenylation and sulfenylation of 3-phenyl quinoxolinones has been developed through N-directed C–H activation in the presence of silver triflimide, and silver carbonate using dichalcogenides ‘on water’. The methodology has been proven to be efficient, regioselective and green. Using this method, a range of selenylations and sulfenylations of the substrates has been carried out in good to excellent yields. Further, late-stage functionalisation produced potential anti-tumour, anti-fungal and anti-bacterial agents making these compounds potential drug candidates.


 Please wait while we load your content...
Please wait while we load your content...