The effective removal of Ni2+, Cd2+, and Pb2+ from aqueous solution by adenine-based nano-adsorbent
Abstract
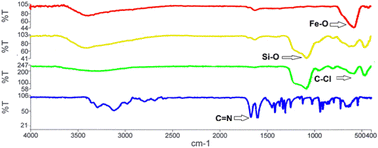
The presence of heavy metal ions in drinking and wastewater generates environmental and human health concerns as they are known as cumulative poisons. Therefore, the purification of contaminated waters is an important ecological issue. Various techniques have been developed to address this issue, where adsorption has received widespread attention. The facile synthesis of effective adenine-based nano-adsorbents is reported and adsorptive removal of Ni2+, Cd2+, and Pb2+ from aqueous media was investigated by inductively-coupled plasma analyses, adsorption isotherms, kinetics, and thermodynamic studies. The effects of pH, adsorbent dose, contact time, and temperature were optimized. The maximum adsorption capacity was achieved at pH = 7, an adsorbent dose of 25 mg, and an initial concentration of 50 mg L−1 at 25 °C. A thermodynamic study showed that adsorption is an endothermic process, and the Langmuir model fitted well to the ion adsorption data to reveal that the maximum adsorption capacities for Ni2+, Cd2+, and Pb2+ were 273.7, 252.4, and 249.8 mg g−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...