Effect of organic solvents on calcium minodronate crystal morphology in aqueous solution: an experimental and theoretical study†
Abstract
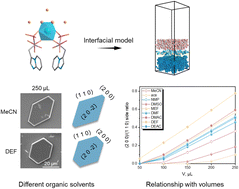
The influence of nine organic solvents on the crystal morphology of calcium minodronate (Ca(Min)2) was investigated by experimental investigations and molecular simulations. Hirshfeld analysis was used to reveal the intermolecular interactions, and the modified attachment energy (AE) model was applied to constructing the Ca(Min)2–organic–water model in different organic–water solvents. The surface structure and the mass density profile were demonstrated and analyzed. The results showed that there were different adsorption conditions in different organic–water solvents. Furthermore, it was found that the (2 0 0)/(1 1 0) side ratio of Ca(Min)2 crystal had a linear relationship with the volume of organic solvent and had a certain correlation with some solvent properties. It is believed that the research developed in this work could have a promising application in prediction of Ca(Min)2 crystal morphology and could give guidance in the selection of organic solvents to control the desirable crystal morphology.


 Please wait while we load your content...
Please wait while we load your content...