N,2,6-Trisubstituted 1H-benzimidazole derivatives as a new scaffold of antimicrobial and anticancer agents: design, synthesis, in vitro evaluation, and in silico studies†
Abstract
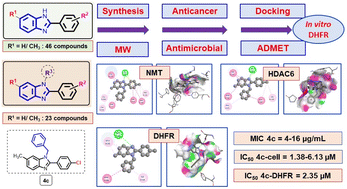
Compounds containing benzimidazole moiety occupy privileged chemical space for discovering new bioactive substances. In continuation of our recent work, 69 benzimidazole derivatives were designed and synthesized with good to excellent yields of 46–99% using efficient synthesis protocol i.e. sodium metabisulfite catalyzed condensation of aromatic aldehydes with o-phenylenediamines to form 2-arylbenzimidazole derivatives followed by N-alkylation by conventional heating or microwave irradiation for diversification. Potent antibacterial compounds against MSSA and MRSA were discovered such as benzimidazole compounds 3k (2-(4-nitrophenyl), N-benzyl), 3l (2-(4-chlorophenyl), N-(4-chlorobenzyl)), 4c (2-(4-chlorophenyl), 6-methyl, N-benzyl), 4g (2-(4-nitrophenyl), 6-methyl, N-benzyl), and 4j (2-(4-nitrophenyl), 6-methyl, N-(4-chlorobenzyl)) with MIC of 4–16 μg mL−1. In addition, compound 4c showed good antimicrobial activities (MIC = 16 μg mL−1) against the bacteria strains Escherichia coli and Streptococcus faecalis. Moreover, compounds 3k, 3l, 4c, 4g, and 4j have been found to kill HepG2, MDA-MB-231, MCF7, RMS, and C26 cancer cells with low μM IC50 (2.39–10.95). These compounds showed comparable drug-like properties as ciprofloxacin, fluconazole, and paclitaxel in computational ADMET profiling. Finally, docking studies were used to assess potential protein targets responsible for their biological activities. Especially, we found that DHFR is a promising target both in silico and in vitro with compound 4c having IC50 of 2.35 μM.


 Please wait while we load your content...
Please wait while we load your content...