Effect of non-covalent self-dimerization on the spectroscopic and electrochemical properties of mixed Cu(i) complexes†
Abstract
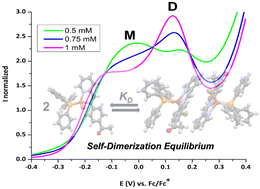
A series of six new Cu(I) complexes with ([Cu(N-{4-R}pyridine-2-yl-methanimine)(PPh3)Br]) formulation, where R corresponds to a donor or acceptor p-substituent, have been synthesized and were used to study self-association effects on their structural and electrochemical properties. X-ray diffraction results showed that in all complexes the packing is organized from a dimer generated by supramolecular π stacking and hydrogen bonding. 1H-NMR experiments at several concentrations showed that all complexes undergo a fast-self-association monomer–dimer equilibrium in solution, while changes in resonance frequency towards the high or low field in specific protons of the imine ligand allow establishing that dimers have similar structures to those found in the crystal. The thermodynamic parameters for this self-association process were calculated from dimerization constants determined by VT-1H-NMR experiments for several concentrations at different temperatures. The values for KD (4.0 to 70.0 M−1 range), ΔH (−1.4 to −2.6 kcal mol−1 range), ΔS (−0.2 to 2.1 cal mol−1 K−1 range), and ΔG298 (−0.8 to −2.0 kcal mol−1 range) are of the same order and indicate that the self-dimerization process is enthalpically driven for all complexes. The electrochemical profile of the complexes shows two redox Cu(II)/Cu(I) processes whose relative intensities are sensitive to concentration changes, indicating that both species are in chemical equilibrium, with the monomer and the dimer having different electrochemical characteristics. We associate this behaviour with the structural lability of the Cu(I) centre that allows the monomeric molecules to reorder conformationally to achieve a more adequate assembly in the non-covalent dimer. As expected, structural properties in the solid and in solution, as well as their electrochemical properties, are not correlated with the electronic parameters usually used to evaluate R substituent effects. This confirms that the properties of the Cu(I) complexes are usually more influenced by steric effects than by the inductive effects of substituents of the ligands. In fact, the results obtained showed the importance of non-covalent intermolecular interactions in the structuring of the coordination geometry around the Cu centre and in the coordinative stability to avoid dissociative equilibria.


 Please wait while we load your content...
Please wait while we load your content...